Monday August 6, 2012
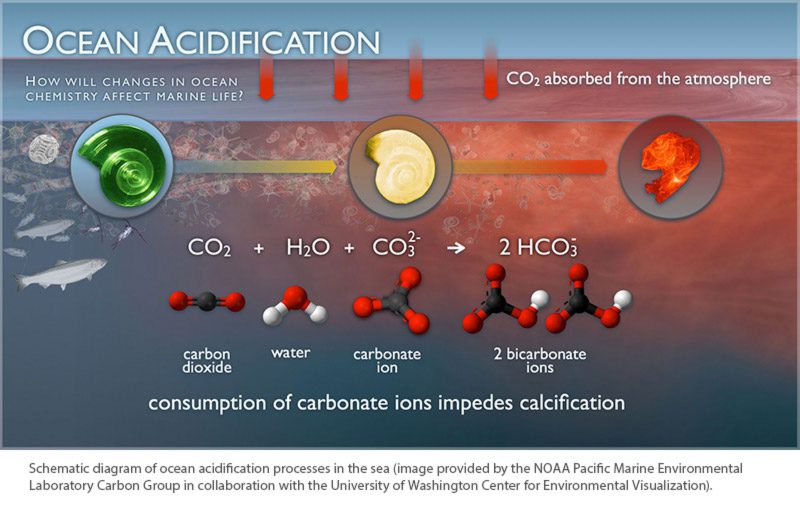
Since the 1970s many people have become familiar with the concept of acid rain and its negative effects on the environment, but fewer are aware of the impacts of fossil fuels on the chemistry of the ocean and the potential consequences on valuable fisheries resources. Ocean acidification is the common term for several chemical reactions that occur when carbon dioxide (CO2) released into the atmosphere is absorbed by seawater. The reactions reduce the carbonate ion concentration and increase the hydrogen ion concentration, making the seawater more acidic. The concentration of carbonate ions in the ocean determines the precipitation of calcium carbonate minerals (e.g., aragonite and calcite), which are used by marine organisms such as oysters and clams, to form their shells or skeletons through calcification. Most ocean surface waters are supersaturated in calcium carbonate minerals, but at lower pH, waters can be undersaturated with these vital minerals.
Since the Industrial Revolution began, there has been a 30% increase in the acidity of the ocean, and based on estimates of carbon dioxide emissions the acidity of the surface waters could rise by 150% by 2100. Recently, a high-resolution computer model was developed to estimate the ocean acidity in the California Current System over the next few decades (Gruber et al. 2012; Figure 1). Results of the simulations indicate that acidification could have considerable impacts on the Pacific Ocean off the West Coast of the U.S. The coast off California is an upwelling zone where winds push surface waters offshore and cause deeper ocean water, naturally high in levels of dissolved CO2, to rise to the surface. The combination of surface waters low in pH (due to upwelling) and aragonite undersaturation makes the California Current System particularly susceptible to ocean acidification.
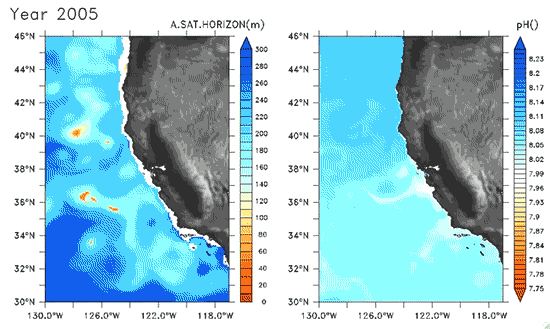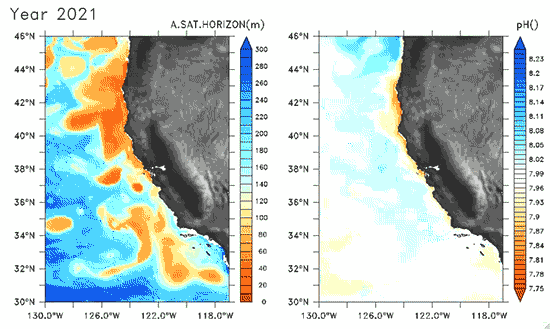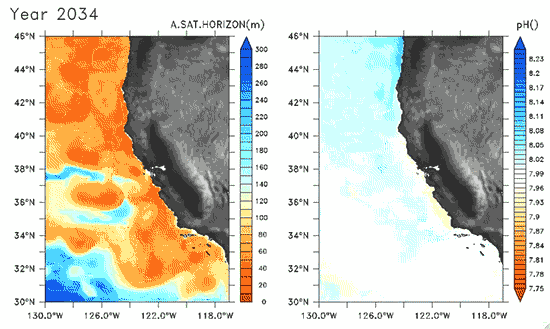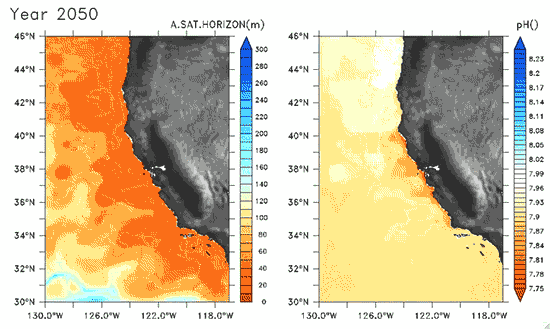
The rise in ocean acidity is clearly a concern for the West Coast shellfish industry, which relies directly on aragonite-secreting organisms such as oysters, but this shift in ocean chemistry could also have significant impacts on West Coast salmon populations. Pteropods are small free-swimming mollusks that form a vital link in marine food webs, and are an important food source for juvenile salmonids in the northern California Current (Brodeur et al. 2007). The shell production of pteropods is sensitive to decreases in pH (Comeau et al. 2010), and at a certain pH, their calcium carbonate shells will actually dissolve (see photos at NOAA). Results of the computer model found that within the next 30 years, long stretches of the California central coast surface waters are projected to be undersaturated in aragonite all summer long, which could have substantial impacts on organisms such as pteropods and their salmonid predators. Researchers are just beginning to understand the potential effects of ocean acidification on the marine ecosystems (Le Quesne and Pinnegar 2012), and each study adds new insight into the complexity of the relationship between water chemistry and ocean food webs.
